The Science Content
-
-
- all matter is made of particles that are too small to see, even with a microscope;
- there is empty space between those particles; and
- the particles are in constant random motion
-
This content aligns with disciplinary core idea PS1.A (Structure and Properties of Matter) at the 5th grade in the NGSS (NGSS Lead States, 2013). The particles we refer to are atoms and molecules, but these terms are not introduced to students at this age (see “Student Language and Teacher Language” below).
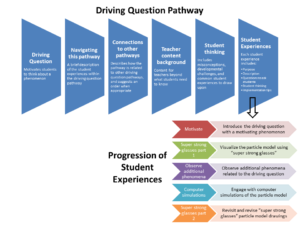
Driving Question Pathways
Each pathway begins with an opportunity for students to express their initial thinking and concludes with an opportunity for students to revisit their initial thinking. We recommend that teachers ask students to keep a record of their initial thinking, for example in a science notebook, so they can compare with their thinking at the end of the pathway.
Click on the image below to view the components of a driving question pathway.
Student Experiences
Each pathway has a sequence of 4–6 “student experiences,” which are well-developed ideas for instructional activities, along with practical guidance for teachers, including:
• the purpose of each student experience;
• class discussion questions;
• relevant aspects of student thinking, such as common misconceptions; and
• implementation tips.
The information provided about student thinking is intended to alert teachers to ideas (both correct and incorrect) their students may have or things to look for in their students’ work/responses.
Within a pathway, student experiences should be done in the sequence presented unless one is marked as optional. The sequence is designed to make students curious and then give them experiences to answer the driving question.
Student Language and Teacher Language
Each pathway includes content background for the teacher. Sometimes this information mentions atoms and molecules, and although these terms are appropriate for teachers, they are probably not appropriate for students. The student experiences and pathways do not attempt to introduce students to atoms and molecules, instead focusing on “particles that are too small to see.” Students may know the terms “atom” and “molecule,” but they probably do not understand their significance. Specifically, an atom is the smallest piece of an element, and a molecule is a combination of two or more atoms. Students should not be discouraged from using the terms, but teachers should not read too much into their use.
The same is true of words like evaporation and condensation, which are used in these resources. Students may, for example, know the word “evaporation” but use it to refer to a liquid disappearing, without understanding the process. We encourage teachers to probe for understanding when students use such words rather than assuming the students understand their meaning.
'Super Strong Glasses'
The particles that matter is made of are too small to see even with a very powerful microscope, so students are asked several times to imagine what they would see with “super strong glasses” that are more powerful than any microscope. We suggest having a microscope or a strong magnifying glass on hand so you can make the point that the particles are too small to see even with these instruments.
Some teachers try to help students engage with imagination by having them cut out and even decorate their own cardboard super strong glasses using cardstock paper (Loughran, Mulhall, & Berry, 2004). Then, anytime an activity calls for students to imagine what they would see with such glasses, the teachers ask students to put them on. We’ve included a template for the cardstock cutouts here. Alternatively, if you have a class set of safety glasses/goggles, you can ask students to wear them and pretend they have “super strong” magnification.
Computer Simulations
The pathways make frequent use of computer simulations that are easy to access via the Internet and are designed for upper elementary students. The simulations are crucial for helping students visualize what they can’t actually see, but we encourage teachers to use them at the appropriate time and not jump ahead to them.
Electronic Scale
Some of the student experiences involve weighing, and it’s important that the process be both straightforward and accurate. We have found that an inexpensive electronic kitchen scale works well. The one we used (http://www.etekcity.com/product/100257.html) sells for about $13 and is available from a wide variety of retailers, including Amazon and Wal-mart.